Mechanistic PK/PD
What is Mechanistic PK/PD?
Mechanistic or semi-mechanistic PK/PD modeling is the addition of biophysics of the therapeutic mechanism of action and relevant target biology to empirical PK/PD modeling. By incorporating biophysics, the model includes enough mechanisms to describe the primary pharmacology of the drug and link it to key biomarkers. In this way, the model distinguishes between biological system-specific and drug-specific parameters, giving it improved extrapolation capabilities to more accurately predict drug efficacy and safety in humans further down the pipeline.
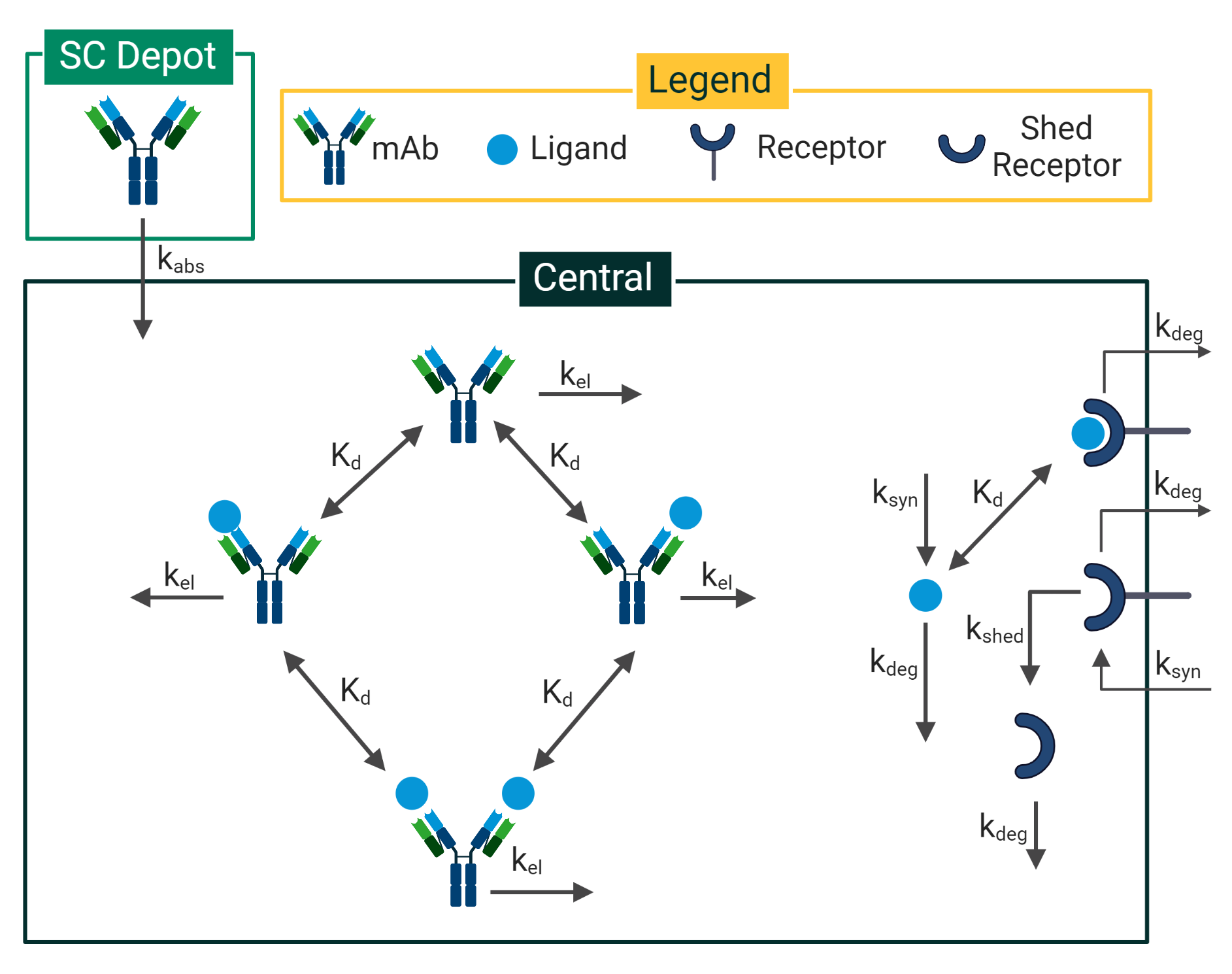
When to use Mechanistic PK/PD Modeling
Mechanistic PK/PD modeling can be used on any modality and throughout the entire spectrum of R&D and provide competitive advantages, as it helps to test and generate hypotheses much more quickly. In the early stages of drug discovery and development, mechanistic PK/PD modeling can leverage the intended pharmacology and data available in the literature to inform questions around dosing feasibility and therapeutic design. In the preclinical stage, mechanistic PK/PD models can incorporate in vitro and in vivo data to inform questions around lead optimization and study design. Translational modeling can be applied to inform first-in-human (FIH) dosing, and once clinical data is available, mechanistic PK/PD models can once again be updated to inform clinical decision making.
Example questions addressed by mechanistic PK/PD models:
- What is the feasibility of our drug concept?
- Comparing to the competitor molecules, will we be able to demonstrate a dosing advantage?
- Will we have a better therapeutic index?
- How can data from a surrogate antibody be used to inform human dosing?
- How can I support optimal dose selection for clinical studies?
- What doses/dosing regimen should we explore in preclinical animal studies?
- What are the optimal drug properties for our drug concept?
- How can I leverage preclinical data to support first-in-human dose selection?
- What are the key knowledge gaps that will impact dose selection?
Mechanistic PK/PD Example
Challenge: Pembrolizumab (Pembro), an anti-PD-1 antibody, is approved for several cancer indications at 200 mg or 2 mg/kg IV every 3 weeks (Q3W). Understanding the percent target receptor occupancy (RO) for PD-1 at this approved dose can help with dose selection for anti-PD-1 combination studies and other anti-PD-1 drugs in development. However, RO can be challenging to determine through direct experimental measurements.
Solution: A semi-mechanistic PKRO model was developed to fit Pembro phase 1 PK data, and the model was used to predict RO.